
ASHP® Injectable Drug Information™
ASHP® Injectable Drug Information™
American Society of Health-System Pharmacists, ASHP®
This online database provides fast, well-arranged, and comprehensive Information about compatibility and stability of injectable drugs.
All relevant data on more than 500 drugs, including coss references to the standard work "AHFS Drug Information".
Detailed answers to frequently asked questions concerning manufacturing, storing, and application of injectible drugs.
Cumulative knowledge of 3,603 resources.
The databases Pediatric Injectable Drugs and Extended Stability for Parenteral Drugs for ASHP Injectable Drugs Information are not included in the offer.
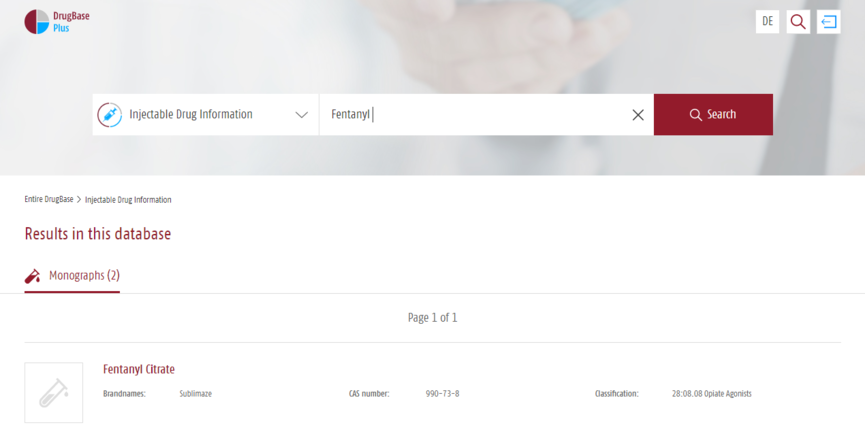
Search entry via active ingredients.
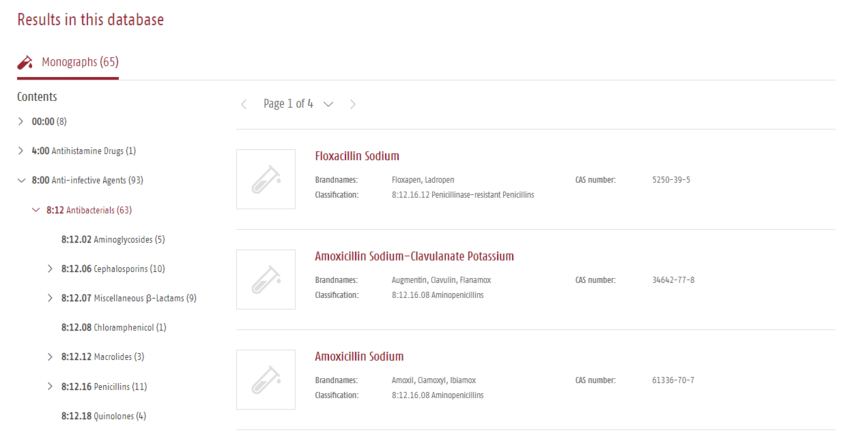
The active ingredients can also be browsed using the AHFS classification system.
Updates
Every 3 months.
Order database
Go to the DAV Media Group store or contact the Databases and Software team for a customized quote (software@dav-medien.de / +49 711 2582-347)
Do you have any questions?
If you have any questions, please do not hesitate to contact us. The DrugBasePlus team will be happy to help you.
Team Databases and Software
+49 711 2582-347
+49 711 2582-347
Mondays to Thursdays
from 9.00 a.m. to 4.30 p.m., Fridays from 9.00 a.m. to 3.30 p.m.
from 9.00 a.m. to 4.30 p.m., Fridays from 9.00 a.m. to 3.30 p.m.