
Fiedler - Encyclopedia of Excipients
Fiedler - Encyclopedia of Excipients
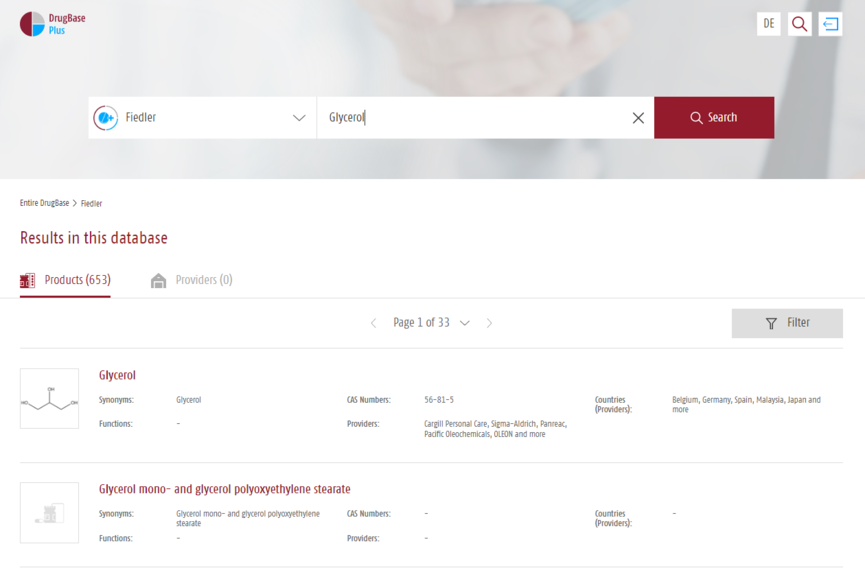
“Fiedler – Encyclopedia of Excipients” is a comprehensive database on excipients used in pharmacy, cosmetics and related fields offering 12,000+ entries. Monographs follow a uniform structure:
- Names and synonyms: INN, INCI, compendial, trivial and chemical names, trademarks, CAS-, EINECS- and E-numbers
- Definition: Description, composition, chemical structure, molar mass
- Basic properties: Appearance, physicochemical data, solubility, spectra, etc.
- Application characteristics and main uses in pharmacy and cosmetics, illustrated by examples
- Stability and incompatibilities
- Pharmacology and toxicology
- Analysis methods
- References with broad literature citations
- List of excipient suppliers
A manufacturers directory gives addresses and full contact details.
Updates
Regular updates
Order database
Go to the DAV Media Group store or contact the Databases and Software team for a customized quote (software@dav-medien.de / +49 711 2582-347)
Available languages
Last update
Fiedler - Encyclopedia of Excipients
Manual
Preface
The „Fiedler - Encyclopedia of Excipients“, first published as a book by H. P. Fiedler in 1971, is now an up-to-date online system, which serves as a unique collection of information about excipients used in pharmacy, cosmetics and related fields. It offers more than 12'000 entries covering all excipients used in dosage forms. There are two different types of monographs:
-
Basic monographs giving general information on the properties of a substance and
-
Trademark monographs giving information on the specific properties and features of a marketed product, produced by a specific company.
Basic monographs refer to trademark monographs and vice versa. Both types of monographs follow the same scheme:
-
Name of the monograph
-
Companies producing the excipient
-
Definition: Pharmacopoeial, INCI and chemical names, synonyms and trademarks, CAS/EINECS number, chemical structure and molecular weight
-
Preparation: Short hint on synthesis or preparation method
-
Properties: Appearance, solubility and density
-
Product data: All other chemical and physico-chemical data available
-
Applications: Main uses in pharmacy and cosmetics, illustrated by examples wherever possible
-
Toxicology: Basic data on toxicology with focus on review articles
-
Analytics: Hints on methods for the determination of the substance
-
References: Literature citations of articles being of interest and not included into the single chapters of the monograph
Trademark monographs normally do not contain data on preparation, toxicology and analytics, because they do not differ from the general description in the corresponding basic monograph. Exceptions are possible, e. g., if there is a product only as one trademark available. The year of the last revision will be added to each monograph.
A manufacturers directory is added, giving the full address, including telephone and fax, as well as the internet an email links of the producer and its affiliates in Europe, North and/or South America and Asia. For each manufacturer the complete list of its excipients is given
In 2010, the “Fiedler - Encyclopedia of Excipients” was taken over by the Wissenschaftliche Verlagsgesellschaft Stuttgart, and from now it will be updated monthly. The authors/editors would like to thank Dr. Klaus G. Brauer, Dr. Eberhard Scholz and Mrs. Pia Rinker for their interest and effort to create a modern electronic data base on pharmaceutical and cosmetic excipients.
Ludwigshafen, Kelkheim and Nürnberg
S. Lang, A. K. Reng and P. C. Schmidt
Abbreviations
The individual excipients are arranged alphabetically. The most customary spelling in scientific literature was chosen. An arrow (▸) means, that the following word is discussed in another section. Registered trademarks (®, TM) are omitted in the encyclopedia for the sake of clarity. This does not mean in any case that the appropriate word is available for common use. Temperature is given in degree Celcius (25° = 25℃) as far as not otherwise denoted.
For reasons of expediency several abbreviations are listed in the text and explained there.
|
° |
℃, Centigrade degrees, Celcius degrees |
|---|---|
|
a |
anno = year |
|
Å |
Angström = 10–7 mm |
|
AAS |
atom absorption spectrometry |
|
ACS |
American Chemical Society |
|
ADI |
acceptable daily intake |
|
ad lib |
ad libitum |
|
aq. |
aqua = water |
|
A.P. |
American patent |
|
APHA |
American Public Health Association (color numbers) |
|
at |
atmosphere (tecn.), unit of pressure, 1 at = 1 kp/cm2 |
|
atm |
atmosphere (physical.), unit of pressure, 1 atm = 1.033 kp/cm2 |
|
AcV |
acetyl value |
|
AV |
acid value |
|
bar |
unit of pressure, 1 bar = 105 Pa |
|
BET |
method for measuring surface area according to Brunauer, Emmett and Teller |
|
BGA |
previous name of the German drug regulatory agency |
|
BIS |
Bibra Information Services (toxicity profiles) |
|
bp |
boiling point |
|
BP |
British Pharmacopeia |
|
BPC |
British Pharmaceutical Codex |
|
cal |
1 calorie = 4.1868 J |
|
CAS |
Chemical Abstracts Service Number |
|
CG |
cosmetic grade |
|
C.I. |
Colour Index (EU) |
|
CIR |
Cosmetic Ingredient Review |
|
cKs |
in the US occasionally taken for cSt |
|
Cod Franc |
Franc Codex Français |
|
comp. |
composition |
|
cP |
centiPoise (mPa∙s) |
|
CPMP |
Committee for Proprietary Medicinal Products of the EMEA |
|
cSt |
centiStoke |
|
CFTA |
Cosmetic, Toiletry, and Fragrance Association |
|
d |
density |
|
dç |
relative density |
|
d |
dies = day |
|
d |
dose |
|
DAB |
German Pharmacopoeia (Deutsches Arzneibuch) |
|
DAC |
German Drug Codex (Deutscher Arzneimittel Codex) |
|
DAS |
published examined German patent application (Deutsche Auslegeschrift) |
|
DBP |
German federal patent (Deutsches Bundespatent) |
|
DGF |
German society for fat science (Deutsche Gesellschaft für Fettwissenschaft) |
|
dil. |
dilutus = diluted |
|
DIN |
German institute for standardization (Deutsches Institut für Normung) |
|
DL |
dosis letalis |
|
DLM |
dosis letalis minima |
|
DRP |
former German patent (Deutsches Reichspatent) |
|
E. |
Engler grades, viscosimetry |
|
EC |
European Community |
|
EINECS |
European Inventory of Existing Chemical Substances |
|
ELINCS |
European List of Notified Chemical Substances |
|
EMEA |
European Medicines Evaluation Agency |
|
E.P. |
English patent |
|
Erg B |
German Pharmacopoeia, Supplement (Ergänzungsband zum Deutschen Arzneibuch) |
|
et al. |
et alii = and others |
|
EV |
ester value |
|
°F |
Fahrenheit degrees, see survey table Temperature Transformation |
|
F Bras |
Bras Farmacopéia das Estados Unidas da Brasil |
|
FCC |
Food Chemicals Codex |
|
FDA |
US Food and Drug Administration |
|
fp |
freezing point |
|
FU |
Farmacopeia Ufficiale della Republica Italiana |
|
G |
giga = 109 |
|
GC |
gas chromatography |
|
GDR |
the former German Democratic Republic |
|
Ger. Offen. (DE) |
published unexamined German patent (DE) documentation; document laid open |
|
GLC |
gas liquid chromatography |
|
GLP |
good laboratory practice |
|
GPC |
gel permeation chromatography |
|
h |
hora = hour |
|
HLB |
hydrophilic-lipophilic balance |
|
HPCE |
high performance capillary electrophoresis |
|
HPLC |
high performance liquid chromatography |
|
i.c. |
intracutaneous |
|
ICH |
International Conference on Harmonization |
|
i.m. |
intramuscular |
|
INCI |
International Nomenclature of Cosmetic Ingredients |
|
int. |
internus = internal |
|
i.p. |
intraperitoneal |
|
IP |
The Pharmacopoeia of India |
|
IR |
infra-red |
|
IRS |
infra-red spectroscopy |
|
I.U. |
international unit |
|
i.v. |
intravenous |
|
IV |
iodine value |
|
J |
Joule, unit of energy, 1 J = m2 kg/s2 (Nm) |
|
JCID |
Japanese Cosmetic Ingredients Dictionary |
|
JP |
The Pharmacopoeia of Japan |
|
JPE |
Japanese Pharmacopoeia Excipients |
|
JSCI |
Japanese Standard of Cosmetic Ingredients |
|
°K |
Kelvin degrees, SI-unit of temperature, 273.15°K = 0℃ |
|
kcal |
kilocalorie(s), unit of energy, 1 kcal = 4.1868 kJ |
|
kJ |
kiloJoule(s), unit of energy, 1 kJ = 0.239 kcal |
|
l |
liter(s), unit of volume 1 l = 1 dm3 |
|
loc. cit. |
loco citato |
|
LC |
liquid chromatography |
|
LD50 |
lethal dose for 50 % |
|
LD lo |
lowest published lethal dose in literature |
|
LOD |
loss on drying |
|
M |
molar, concentration unit e.g. 3M |
|
MAC |
Maximum Admissible Concentration (drinking water) |
|
MAK |
Maximale Arbeitsplatz-Konzentration |
|
max. |
maximum |
|
mCi |
milliCurie |
|
µCi |
microCurie |
|
MEL |
Maximum Exposure Limits |
|
meq |
milliequivalents/g |
|
mg% |
milligramm percent = mg of dissociated material in 100 g total mass (solvent) |
|
min |
minute(s) |
|
min. |
minimal, minimum |
|
MITI |
Ministry of International Trade and Industry (Japan) |
|
MLD |
minimum lethal dose |
|
mmHg |
millimeter(s) of mercury, unit of pressure, 1 mmHg = 133.322387415 Pa |
|
Mn |
number molecular weight, determined for polymers by osmometry |
|
mol |
mole(s), mass of 6.023∙1023 moles (g) |
|
molwt |
molecular weight |
|
mp |
melting point |
|
mp* |
melting point according to pharmacopoeial 'open capillary method' |
|
mPa∙s |
milliPascal seconds, unit of viscosity, 1 mPa∙s = 1 cP |
|
MS |
mass spectroscopy |
|
mth |
month |
|
Mw |
weight molecular weight, determined for polymers by light scattering or ultracentrifugation |
|
n. |
normal |
|
n20 |
number of members of a statistical collective |
|
nD |
refractive index |
|
NF |
The US National Formulary |
|
nm |
nanomolar |
|
nm |
nanometer(s), unit of length, 1 nm = 10-9 m |
|
nmol |
nanomole(s) = 10-9 mol |
|
NND |
New and Nonofficial Drugs |
|
NNR |
New and Nonofficial Remedies |
|
NRF |
new prescription formulary (Neues Rezept-Formularium) |
|
NTP |
US National Toxicological Program |
|
NV |
neutralization value |
|
ÖAB |
Austrian Pharmacopoeia (Österreichisches Arzneibuch) |
|
OECD |
Organization for Economic Co-operation and Development |
|
OEL |
Occupational Exposure Limits |
|
OHV |
hydroxyl value |
|
OS |
see Ger. Offen. |
|
OVIs |
organic volatile impurities |
|
O/W |
oil-in-water (emulsion) |
|
P |
Poise, 1 P = 0.1 Pa∙s, dynamical viscosity |
|
P. |
granted patent, does not indicate that the patent is still valid at present |
|
Pa |
Pascal, unit of pressure, 1 Pa = m-1 kg s-2 (Nm-2) |
|
PC |
paper chromatography |
|
PDA |
Permitted Daily Exposure (Residual Solvents) |
|
pH |
the negative logarithm of the hydrogen ion concentration |
|
Ph Belg |
Pharmacopée Belge |
|
Ph Dan |
Pharmacopoea Danica |
|
Ph F |
Pharmacopoea Fennica |
|
Ph Franc |
Pharmacopoée Française |
|
Ph Helv |
Pharmacopoea Helvetica |
|
Ph Ned |
Pharmacopoea Nederlandica |
|
Ph Nord |
Pharmacopoea Nordica |
|
Ph Norv |
Pharmacopoea Norvegica |
|
Ph Svec |
Pharmacopoea Svecica |
|
p.i. |
post injectionem, infusionem = after injection, infusion |
|
pM |
picomolar |
|
p.o. |
per os = oral |
|
PON |
peroxide number |
|
ppb |
parts per billion |
|
ppm |
parts per million |
|
prim. |
primary |
|
PV |
peroxide value |
|
RD |
Recognised Disclosure numbers of CTFA |
|
RH |
relative humidity |
|
RhV |
Rhodane value |
|
ROI |
residue on ignition |
|
RPHPLC |
reversed phase high pressure liquid chromatography |
|
s |
second(s) |
|
s.c. |
subcutaneous |
|
SDA |
specially denaturated alcohol |
|
sec. |
secondary |
|
SEM |
scanning electron microscope |
|
sp |
solidification point |
|
SSU |
Saybolt universal second |
|
St. |
Stokes, 1 St. = 10−4 m2 s−1, kinematic viscosity |
|
SV |
saponification value |
|
t |
time |
|
TD lo |
lowest published toxic dose in literature |
|
tert. |
tertiary |
|
TGA |
Therapeutic Goods Administration (Australia) |
|
TLC |
thin layer chromatography |
|
TLV |
Threshold Limit Value |
|
TSCA |
Toxic Substances Control Act |
|
U. |
units |
|
Ung. |
unguentum |
|
USP |
The United States Pharmacopoeia |
|
vol. |
volume |
|
vol-% |
volume percent = 1 ml of dissociated material in 100 ml solution |
|
W/O |
water-in-oil (emulsion) |
|
wt |
weight |
Units of Measurements
As a result of international agreements a unification of the measurement system has been achieved (International System of Units; Système international = SI-units). In Germany the “Law on Units in the Measurement System” was passed on 2 July 1969, the implementing ordinance concerning this Law was passed on 26 July 1970 and a Law for amending the Law on Units in the Measurement System on 6 July 1973. According to this legal framework the unitsdefined in these laws and regulations are obligatory for business and official transactions. Details have been defined in DIN 1301 “Units, unit names, unit symbols” (DIN Deutsches Institut für Normung e. V., Burggrafenstraße 6, 10787 Berlin, Tel.: +49 30 2601-0, Fax: +49 30 2601-1231 and in ISO 31, which is now superseded by the harmonized ISO/IEC 80000 standard (ISO copyright office, Case postale 56, CH-1211 Geneva 20, Tel. + 41 22 749 01 11, Fax + 41 22 749 09 47.
In the USA the same units etc. were first described in The Metric Conversion Act of 1975 (Public Law 94–168) (see also G.G. Stoner, Cosmet. Toiletries 93, No. 11, 57 [1978]) and are now harmonized in ISO 80000.
The new units have been taken into account in the present edition of the Handbook, when new documentation has been provided by the manufacturers of auxiliary substances. The new units have also been used in some revisions and additions by the editors. However, in the following the most important changes in the measurement system are given so that conversions from older unit systems may be performed, if required.
There are nine basic SI units, of which the following 7 are relevant:
SI-Basic Units
|
Basis physical quantity |
Basic unit |
|
|---|---|---|
|
Name |
Symbol |
|
|
length |
meter |
m |
|
time |
second |
s |
|
mass |
kilogram |
kg |
|
temperature |
kelvin |
K |
|
electrical current |
ampere |
A |
|
luminous intensity |
candela |
cd |
|
amount of substance |
mole |
mol |
17 further units are derived from the nine basic units, of which the following derived SI units, with their names,are relevant:
|
Physical quantity |
Unit |
Expressed as basic units or derived units |
|
|---|---|---|---|
|
Name |
Symbol |
||
|
work, energy, quantity of heat |
joule |
J |
1J = 1Nm = 1(kg*m2)/s2 = 1Ws |
|
illuminance |
lux |
lx |
1lx = 1 lm//m2 |
|
force |
Newton |
N |
1N = 1 (kg*m)/s2 |
|
pressure |
Pascal |
Pa |
1 Pa = 1N/m2 = 1kg/(m*s2) |
The following prefixes were established for decimal multiples and subdivisions of units:
|
Multiples |
Prefix |
Symbol |
Subdivisions |
Prefix |
Symbol |
|---|---|---|---|---|---|
|
101 |
deca |
da |
10–1 |
deci |
d |
|
102 |
hecto |
h |
10–2 |
centi |
c |
|
103 |
kilo |
k |
10–3 |
milli |
m |
|
106 |
mega |
M |
10–6 |
micro |
µ |
|
109 |
giga |
G |
10–9 |
nano |
n |
|
1012 |
tera |
T |
10–12 |
pico |
p |
|
1015 |
peta |
P |
10–15 |
femto |
f |
|
1018 |
exa |
E |
10–18 |
atto |
a |
Spelling conventions for SI Units
If derived units consist of several basic units in the numerator or denominator, these are written one after the other with some space in between (in the case of a typewriter a single space).
Examples: N s/m, not Ns/m; kg m/s, not kgm/s.
However, multiples or subdivisions are written together with the respective units.
Examples: MNm, not M N m; mm2/s, not m m2/s.
Indices must not be appended to unit symbols but should be added to the formula symbol giving the physical quantity. Hence e.g. the normal volume may no longer be given as Nm3 (normal cubic meter) but instead as: normal volume Vn=…m3 or better: volume V=…m3 in the normal state.
Variations are permissible in the names for physical quantities, but the standard form of the unit name should always be used. This principle helps to avoid misleading spellings.
Data for the various units relevant to the present Handbook, or the corresponding conversion factors for previously used units, are given in the following table:
Legal Units
|
Physical quantity |
Legal units |
|
Conversion |
Comment |
|||
|---|---|---|---|---|---|---|---|
|
Symbol |
SI Unit |
Relationship |
Recommended unit |
Previous unit |
|||
|
Name |
Symbol |
||||||
|
mass |
m |
kilogram |
kg |
1g = 10-3kg; 1t = 103kg |
kg |
|
SI basic unit |
|
density |
ρ |
kilogram per cubic meter |
kg/m3 |
|
g/cm3; kg/m3 |
|
|
|
specific volume |
V |
cubic meter per kilogram |
m3/kg |
|
cm3/g; m3/kg |
|
|
|
amount of substance |
n |
mole |
mol |
|
kmol |
|
|
|
mass related to amount of substance (molar mass) |
M |
kilogram per mol |
kg/mol |
|
kg/kmol |
|
|
|
volume related to amount of substanc (molar volume) |
Vm |
cubic meter per mole |
m3/mol |
|
m3/kmol |
|
|
|
surface tension and interfacial tension |
|
milli-Newton per meter |
mN/m |
|
mN/m |
dyn/cm |
for all liquids |
|
thermodynamic or kelvin temperature, celsius temperature |
T, Θ, t, ϑ |
Kelvin |
K |
t = T -273K |
K ℃ |
°K |
SI basic unit practical conversion: t = T -273K |
|
dynamic viscosity |
η |
Pascal second |
Pa·s |
|
mPa·s |
cP (centipoise) |
|
|
kinematic viscosity |
ν |
square meter per second |
m2/s |
|
cm2/s |
st (stokes) |
|
Conversion of the most common British (UK) and American (US) Units into SI Units
|
Physical quantity |
Name of the unit |
Symbol |
Conversion into SI Units |
|
|---|---|---|---|---|
|
length |
inch |
in |
1in |
=25.4mm |
|
foot |
ft |
1ft=12in |
=0.3048m |
|
|
yard |
yd |
1yd=3ft |
=0.9144m |
|
|
mile (statute) |
|
1mile=1760yd |
=1.609344km |
|
|
nautical mile (intern.) |
n. mile |
1n. mile |
=1.852km |
|
|
area |
square inch |
sq in |
1sq in |
=6.4516cm2 |
|
square foot |
sq ft |
1sq ft=144sq in |
=929.030cm2 |
|
|
square yard |
sq yd |
1sq yd=9sq ft |
=0.836127m2 |
|
|
rood |
|
1rood=1210sq yd |
=1011.71m2 |
|
|
acre |
|
1acre=4roods |
=4046.86m2 |
|
|
square mile |
sq mile |
1sq mile=640acres |
=2.589988km2 |
|
|
volume |
cubic inch |
cu in |
1cu in |
=16.3871cm3 |
|
cubic foot |
cu ft |
1cu ft |
=28.3168dm3 |
|
|
cubic yard |
cu yd |
1cu yd |
=0.764555m3 |
|
|
British measure of capacity |
UK fluid ounce |
UK fl oz |
1fl oz |
=28.4131cm3 |
|
UK gill |
|
1gill=5fl oz |
=0.142065dm3 |
|
|
UK pint |
UK pt |
1pt=20fl oz |
=0.568261dm3 |
|
|
UK quart |
UK qt |
1qt=2pt |
=1.13652dm3 |
|
|
UK gallon |
UK gal |
1gal=4qt |
=4.54609dm3 |
|
|
American measure of capacity (liquid) |
US fluid ounce |
US fl oz |
1fl oz |
=29.5735cm3 |
|
US gill |
gi |
1gi=4fl oz |
=0.118294dm3 |
|
|
US liquid pint |
liq pt |
1liq pt=4gi |
=0.473176dm3 |
|
|
US liquid quart |
liq qt |
1liq qt=2liq pt |
=0.946353dm3 |
|
|
US gallon |
US gal |
1gal=4liq qt |
=3.78541dm3 |
|
|
US barrel (oil) |
bbl |
1bbl=42gal |
=158.987dm3 |
|
|
American measure of capacity (dry) |
US dry pint |
dry pt |
1dry pt |
=0.550610dm3 |
|
US dry quart |
dry qt |
1dry qt=2dry pt |
=1.10122dm3 |
|
|
US peck |
pk |
1pk=8dry qt |
=8.80976dm3 |
|
|
US bushel |
bu |
1bu=4pk |
=35.2391dm3 |
|
|
mass |
grain |
gr |
1gr |
=0.064799g |
|
dram (avoir dupois) |
dr |
1dr=27.34375gr |
=1.77185g |
|
|
ounce (avdp.) |
oz |
1oz=16dr |
=28.3495g |
|
|
troy ounce |
oz tr |
1oz tr=480gr |
=31.1035g |
|
|
pound (avdp.) |
lb |
1lb=16oz |
=0.453592kg |
|
|
troy pound |
lb tr |
1lb tr=12oz tr |
=0.373242kg |
|
|
stone (UK) |
|
1stone=14lb |
=6.35029kg |
|
|
hundredweight (UK) |
cwt |
1cwt=112lb |
=50.8023kg |
|
|
(long) ton (UK) |
ton |
1ton=2240lb |
=1016.05kg |
|
|
Shorthundredweight (US) |
sh cwt |
1sh cwt=100lb |
=45.3592kg |
|
|
short ton (US) |
sh ton |
1sh ton=2000lb |
=907.185kg |
|
|
force |
poundal |
pdl |
1pdl=1lb ft/s2 |
=0.138255N |
|
pound-force |
lbf |
1lbf |
=4.44822N |
|
|
UK ton-force |
UK tonf |
1tonf=2240lbf |
=9964.02N |
|
|
US ton-force=2kip |
US tonf |
1tonf=2000lbf |
=8896.44N |
|
|
pressure |
pound-force / sq ft |
lbf / ft2 |
1lbf / ft2 |
=47.8803Pa |
|
pound-force / sq in (p.s.i.) |
lbf / in2 |
1lbf / in2 |
=6.89476kPa |
|
|
energy, quantity of heat |
foot pound-force |
ft lbf |
1ft lbf |
=1.35582J |
|
British thermal unit |
Btu |
1Btu |
=1.05506kJ |
|
|
therm |
|
1therm=105 Btu |
=105.506MJ |
|
|
power |
British thermal unit / hour |
Btu / h |
1Btu / h |
=0.293071W |
|
horsepower |
hp |
1hp=550ft lbf / s |
=745.700W |
|
|
temperature |
degree Fahrenheit |
°F |
temp. in ℃ =(temp. in °F −32)·5/9 |
∆1°F=∆5/9℃ |
Sieves
Sieves are used to classify powders and granules in production and for analytical purposes. They are specified according to DIN/ISO 565 which is equivalent to ISO 3310-1. The following table, which is based on the USP text, compares the ISO sieves with those mentioned in Ph. Eur. and Japan. The US sieve numbers (mesh) are provided in the table for conversion purposes only.
|
ISO Nominal Aperture |
|
|
|
||
|---|---|---|---|---|---|
|
Principal sizes |
Supplementary sizes |
Recommended USP sieves |
US sieve no. (mesh) |
Ph. Eur. sieve no. |
Japan sieve no. |
|
11.20mm |
|
|
|
11200 |
|
|
5.60mm |
|
|
|
5600 |
3.5 |
|
|
4.75mm |
|
|
|
4 |
|
4.00mm |
|
4000 |
5 |
4000 |
4.7 |
|
|
3.35mm |
|
6 |
|
5.5 |
|
2.80mm |
|
2800 |
7 |
2800 |
6.5 |
|
|
2.36mm |
|
8 |
|
7.5 |
|
2.00mm |
|
2000 |
10 |
2000 |
8.6 |
|
|
1.7mm |
|
12 |
|
10 |
|
1.40mm |
|
1400 |
14 |
1400 |
12 |
|
|
1.18mm |
|
16 |
|
14 |
|
1.00mm |
|
1000 |
18 |
1000 |
16 |
|
|
850µm |
|
20 |
|
18 |
|
710µm |
|
710 |
25 |
710 |
22 |
|
|
600µm |
|
30 |
|
26 |
|
500µm |
|
500 |
35 |
500 |
30 |
|
|
425µm |
|
40 |
|
36 |
|
355µm |
|
355 |
45 |
355 |
42 |
|
|
300µm |
|
50 |
|
50 |
|
250µm |
|
250 |
60 |
250 |
60 |
|
|
212µm |
|
70 |
|
70 |
|
180µm |
|
180 |
80 |
180 |
83 |
|
|
150µm |
|
100 |
|
100 |
|
125µm |
|
125 |
120 |
125 |
119 |
|
|
106µm |
|
140 |
|
140 |
|
90µm |
|
90 |
170 |
90 |
166 |
|
|
75µm |
|
200 |
|
200 |
|
63µm |
|
63 |
230 |
63 |
235 |
|
|
53µm |
|
270 |
|
282 |
|
45µm |
|
45 |
325 |
45 |
330 |
|
|
38µm |
|
|
38 |
391 |
|
|
|
|
|
|
|
References
USP/NF (786) Particle size distribution estimation by analytical sieve testing, USP32/NF27, 307 (2009).
Ph. Eur. 2.1.4 Sieves, Kommentar Ph. Eur. 4.00, 2.1.4 Siebe (2004).
J. Goede, Verarbeitung von Stoffen/Trennen, in E. Nürnberg and P. Surmann (eds.), Hagers Handbuch der Pharmazeutischen Praxis, 5th ed., vol. 2 Methods, p. 586-587, Springer, Berlin, Heidelberg, New York (1991).
EU-Declaration of Excipients
Guideline on the Excipients in the Label and Package Leaflet of Medicinal Products for Human Use
Volume 3B Guidelines, July 2003
Pursuant to Article 65 of European Council Directive 2001/83/EC
Annex: Excipients and Information for the Package Leaflet
www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003412.pdf
Explanatory Notes: The Annex is structured as follows:
Name: This is the name of the excipient using INN or PhEur nomenclature where possible, including a reference to E-numbers where relevant.
Route of administration: This is necessary because the information may depend upon the route of administration, e.g. for benzalkonium chloride the information relating to bronchospasm is relevant only for the respiratory route.
Threshold: It is accepted that excipients may only show an effect above a certain ‘dose’.
Except where otherwise stated, thresholds are expressed as Maximum Daily Doses of the excipient in question, taken as part of a medicinal product.
The threshold is a value, equal to or above which it is necessary to provide the information stated.
A threshold of ‘zero’ means that it is necessary to state the information in all cases where the excipient is present in the medicinal product.
Information for the Package Leaflet: The information is presented here in a simple form, in clear and understandable terms for the patient.
The text often refers to the term ‘per dose’ meaning dose of the medicinal product.
Since doses may be extremely variable, applicants must take into account the maximum single dose of the medicinal product, as defined in the SPC, Section 4.2.
For this reason the information sometimes contains the expression ‘up to x mg per dose’, for example.
If the pharmaceutical form is a solid form, e.g. tablet, capsule, suppository, powder in a sachet, it may be better to refer to the amount per tablet, capsule etc.
Cosmetic Ingredient Review (CIR)
The Cosmetic Ingredient Review was established in 1976 by the industry trade association (then the Cosmetic, Toiletry, and Fragrance Association, now the Personal Care Products Council), with the support of the U.S. Food and Drug Administration and the Consumer Federation of America. Although funded by the Council, CIR and the review process are independent from the Council and the cosmetics industry.
The Cosmetic Ingredient Review thoroughly reviews and assesses the safety of ingredients used in cosmetics in an open, unbiased, and expert manner, and publishes the results in the open, peer-reviewed scientific literature.
Cosmetic Ingredient Review
1101 17th Street N. W., Suite 412
Washington DC 20036-4702 (USA)
Tel. +1-202-331-0651
Fax +1-202-331-0088
Further informations: Publications, ingredient reports, quick reference table: www.cir-safety.org/publications.shtml
Toxicity Profiles
The BIBRA Toxicity Profiles are critical reviews of the most pertinent toxicological data published on commercially important chemicals. Prepared by experienced toxicologists, each Profile is compiled principally from primary sources, as a comprehensive yet succinct evaluative summary. The Toxicity Profile project has been running for over 20 years, and BIBRA has now built up a formidable series of monographs covering neraly 500 chemicals.
Products an services, Toxicity profiles, search for specific profiles, list of all profiles avaible: www.bibra-information.co.uk
Residual Solvents
Note for Guidance on Impurities: Residual Solvents
The European Agency for the Evaluation of Medicinal Products Human Medicines Evaluation Unit
CPMP/ICH/283/95, March 1998, ICH Harmonised Tripartite Guideline Q3C
The limits for residual solvents given in Ph Eur, USP and JP are based on the Guidelines for Residual Solvents (CPMP/ICH/283/95, March 1998) which were adopted by the International Conference on Harmonization (ICH) for registration of pharmaceuticals for human use and prescribe limits for the content of solvents which may retain in active substances, excipients and medicinal products after processing. The residual solvents are listed in Appendix 1 by common names and structures. They were evaluated for their possible risk to human health and placed into one of three classes as follows:
Class 1 solvents: Known human carcinogens, strongly suspected human carcinogens, and environmental hazards. They are listed in Table 1.
Class 2 solvents (solvents to be limited): Non-genotoxic animal carcinogens or possible causative agents of other irreversible toxicity such as neurotoxicity or teratogenicity. They are listed in Table 2.
Class 3 solvents (solvents with low toxic potential): Solvents with low toxic potential to man; no health-based exposure limit is needed. Class 3 solvents have PDEs of 50mg or more per day. They are listed in (Definition of PDE: Permitted daily exposure).
Further information: www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002674.pdf and www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/general/general_content_000431.jsp&jsenabled=true
Limits for Drinking Water
Water legislation in the EU is based on the Council Directive 98/83/EC of November, 3, 1998 on the quality of water intended for human consumption; Official Journal L 330, 05/12/1998, p.0032-0054.
Parameters and parametric values are presented in Annex I: eur-lex.europa.eu/LexUriServ/LexUriServ.do
Coloring Agents
Coloring Agents for Cosmetic Products in the USA, Japan and EU
The coloring of cosmetic products is regulated by law in the USA, Japan and the European Union. Other countriesoften use the regulations of these countries as a model. The following references can be used for further information.
Blue List: Cosmetic Ingredients, ECV Editio Cantor Verlag, Aulendorf (2000); G. Otterstätter, Die Färbung von Lebensmitteln, Arzneimittel, Kosmetika, Behr's Verlag, Hamburg (1995); C. Fox, Color in Cosmetics, Cosmetics & Toiletries 111 (3), 35 (1996); G. Otterstätter, Die Färbung von Kosmetika, SÖFW-Journal 123, 328 (1997); G. Otterstätter, Kosmetische Färbemittel im internationalen Vergleich, Parfümerie und Kosmetik 78 (10), 8 (1997); R. Romanowski and R. Schüeller, Creating Colorful Cosmetics, Cosmetics & Toiletries 112 (9), 73 (1997); D. C. Steinberg, Regulatory Review, Cosmetics & Toiletries 111 (10), 29 (1996); CTFA Color Handbook 1992, Cosmetic, Toiletry and Fragrance Association, Inc., 1101 17th Street, NW, Suite 300, Washington, D.C. 20036-4702, USA; Principles of cosmetic licensing in Japan, 2nd Edition; Yakuji Nippo Ltd. 1, Kanda Izumicho, Chiyoda-Ku, Tokyo, 101, Japan.
The coloring agents authorized in the USA for coloring cosmetic products are listed in the Code of Federal Regulations 21 (CFR 21), which can be ordered from the U.S. Government Printing Office, Superintendent of Documents, Mail Stop: SSOP. Washington, DC 20402-9328.
Furthermore a summary of color additives listed for use in the United States in foods, drugs, cosmetics, and medical devices is given by the following address: Office of Food Additive Safety (HFS-200), Center for Food Safety and Applied Nutrition, Food and Drug Administration, 5100 Paint Branch Parkway, College Park, MD 20740-3835, USA.
Further information: ec.europa.eu/consumers/cosmetics/cosing/index.cfm
Coloring Agents for Cosmetic Products in Germany
www.gesetze-im-internet.de/bundesrecht/kosmetikv/gesamt.pdf
Coloring Substances in Drugs
The use of coloring substances in medicaments is regulated by the Council Directive 94/36/EEC, June 30, 1994, originally designed for colors in food. It is completed by the Council Directive 95/45/EEC, July 26, 1995 on purity of food colors. The list (Appendix I) contains the following substances:
Appendix I
|
Colour shade EEC No. |
Name |
Chemical name or description |
|---|---|---|
|
Yellow |
||
|
E 100 |
Curcumin |
1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadien-3,5-dione |
|
E 101 |
Riboflavin (Lactoflavin) |
7,8-dimethyl-10-(1'-D-ribityl)isoalloxazin |
|
E 102 |
Tartrazine |
5-hydroxy-1-(4-sulfophenyl)-4-(4-sulfophenylazo)-3-pyrazol-carboxylic acid, trisodium salt |
|
E 104 |
Quinoline yellow |
2-(1,3-dioxo-2-indanyl)quinoline disulfonic acid, disodiumsalt (also containing monosulfonic acid derivatives, also partly methylated) |
|
Orange |
||
|
E 110 |
Yellow orange S (Sunset yellow FCF) |
6-hydroxy-5-(4-sulfophenylazo)-2-naphthalene-sulfonic acid, disodium salt |
|
Red |
||
|
E 120 |
Carmine (Cochineal carminic acid) |
extract from Dactylopius coccus [syn. Coccus cacti] including the ammonium salts |
|
E 122 |
Azorubin (Carmoisine) |
1'-hydroxy-1,2'-azonaphthalene-4,4'-disulfonic acid, disodium salt |
|
E 123 |
Amaranth |
2-hydroxy-1,1'-azonaphthalene-3,4',6-trisulfonic acid, trisodium salt |
|
E 124 |
Ponceau 4R (Cochineal Red A) |
2-hydroxy-1,1'-azonaphthalene-4',6,8-trisulfonic acid, trisodium salt |
|
E 127 |
Erythrosine |
2',4',5',7'-tetraiodofluorescein, disodium salt or 2-(6-hydroxy-2,4,5,7-tetraiodo-3-oxo-3H-xanthene-9-yl)benzoic acid, disodium salt |
|
E 128 |
Red 2G |
disodium-8-acetamido-1-hydroxy-2-phenylazo-naphthalene-3,6-disulfonate |
|
E 129 |
Allulared AC |
disodium-2-hydroxy-1(2-methoxy-5-methyl-4-sulfo-phenyl- azo)-naphthalene-6-sulfonate |
|
Blue |
||
|
E 131 |
Patent Blue V |
α-(4-diethylaminophenyl)-α-(4-diethylimino-2,5-cyclohexadiene-1-ylidene)-5-hydroxy-4-sulfo-o-toluenesulfonate, calcium salt |
|
E 132 |
Indigo carmine (Indigotin) |
3,3'-dioxo[∆2,2'-biindoline]-5,5'-disulfonic acid, disodium salt |
|
E 133 |
Brilliant Blue FCF |
disodium-α-(4-(N-ethyl-3-sulfobenzylamino)phenyl)-α-(4(N-ethyl-3-sulfobenzylamino)cyclohexa-2.5-diethylidene) toluene-2-sulfonate |
|
Green |
||
|
E 140 |
Chlorophyll |
Chlorophyll a and Chlorophyll b |
|
E 141 |
Chlorophyll- and Chlorophyllin- copper-complexes |
Chlorophyll a(b)-copper-complexes and Chlorophyllin a(b)-copper-complexes |
|
E 142 |
Acid brilliant green BS (Wool GreenBS, Lissamine green) |
1-(α-(4-dimethylimino-2,5-cyclohexadiene-1-ylidene)-4-dimethylaminobenzyl)-2-hydroxy-6-sulfo-3-naphthalenesulfonate, sodium salt |
|
Brown |
||
|
E 150 |
Caramel |
Product manufactured from sucrose (or other kinds of sugar suitable for consumption) solely by heating, or amorphous, brown, water-soluble products manufactured by controlled heating of edible sugars in the presence of acetic, citric, phosphoric or sulfuric acid, sulfur dioxide, ammonium-, sodium- and potassium hydroxide, -carbonate, -phosphate, -sulfate or -sulfite. |
|
|
a) Simple caramel |
Prepared by controlled heating of carbohydrates. |
|
|
b) Sulfite caramel |
Prepared by controlled heating of carbohydrates under the addition of sodium or potassium sulfite or bisulfite. |
|
|
c) Ammonia caramel |
Prepared by controlled heating of carbohydrates under the addition of ammonia compounds like ammonium hydroxide, ammonium hydrogen carbonate or ammonium carbonate. |
|
|
d) Ammonium sulfite caramel |
Prepared by controlled heating of carbohydrates under the addition of sulfite and ammonia compounds. |
|
E 154 |
Brown FK |
A mixture of the following main components: I. sodium-4-(2,4-diaminophenylazo)benzene sulfonate II. sodium-4-(4,6-diamino-m-tolylazo)benzene sulfonate III. disodium-4,4'-(4,6-diamino-1,3-phenylenebisazo)-di-benzenesulfonate IV. disodium-4,4'-(2,4-diamino-1,3-phenylenebisazo)-di-benzenesulfonate V. disodium-4,4'-(2,4-diamino-5-methyl-1,3-phenylenebisazo)-di-benzenesulfonate VI. trisodium-4,4',4''-(2,4-diaminobenzene-1,3,5-trisazo) tribenzenesulfonate |
|
|
Brown HT |
disodium-4,4'-(2,4-dihydroxy-5-hydroxymethyl-1,3-phenylenebisazo)-di-naphthalene-1 sulfonate |
|
Black |
||
|
E 151 |
Brilliant Black BN |
4-sulfophenylazo-4-(7-sulfonaphthalene)-1-azo-2-(8-acet- amido-1-hydroxy-3,5-naphthalenedisulfonic acid), tetrasodium salt |
|
E 153 |
Carbon Black (Carbo medicinalis vegetabilis) |
Vegetable charcoal having the properties of medicinal char coal |
|
Various colours |
||
|
E 160 |
Carotinoids: |
|
|
|
a) β,ε-Carotene (α''-Carotene) Betacarotene (β,β-Carotene) β,ψ -Carotene (γ-Carotene) |
all-trans forms as main components |
|
|
b) Bixin, Norbixin (Annatto, Orlean) |
Annatto is an extract of the seeds of Bixa orellana; the extract in oil is the carotinoid Bixin, the mono methyl ester of 6,6'-diapo-6,6'-carotene-dioic acid. Norbixin is the free dicarboxylic acid; the main colouring substance of the aqueous Annatto extracts is the alkali salt of Norbixin. |
|
|
c) Capsanthin, Capsorubin |
Extract from paprika (Capsicum annuum fruits) |
|
|
d) Lycopene |
ψ,ψ-Carotene (all-trans-form as main constituent) |
|
|
e) 8'-Apo-β,ψ-carotenal |
8'-apo-β,ψ-carotenal (all-trans-form as main constituent) |
|
|
f) Ethyl-8'-apo-β,ψ-carotenoate |
ethyl-8'-apo-β,ψ-carotenoate (all-trans-form as main constituent) |
|
E 161 |
Xanthophylls: |
Xanthophylls are keto- and/or hydroxy-derivatives of carotenes |
|
|
a) Flavoxanthin |
5,8-epoxy-5,8-dihydro-β,β-carotene-3,3'-diol |
|
|
b) Lutein |
β,ε-carotene-3,3'-diol |
|
|
c) Cryptoxanthin |
β,β-carotene-3-ol |
|
|
d) Rubixanthin |
β,ψ-carotene-3-ol |
|
|
e) Violaxanthin |
5,6,5',6'-diepoxy-5,5',6,6'-tetrahydro-β,β-carotene-3,3'-diol |
|
|
f) Rhodoxanthin |
4',5'-didehydro-4,5-retro-β,β-carotene-3,3'-dione |
|
|
g) Canthaxanthin |
β,β-carotene-4,4'-dione |
|
E 162 |
Beetroot Red, Betanin |
aqueous extract from the root of the red beet (Beta vulgaris var. conditiva) |
|
E 163 |
Anthocyanins |
Anthocyanins are glycosides of hydroxylated derivatives of2-phenylbenzopyrylium salts; they contain the following anthocyanidins as aglycones: Pelargonidin, Cyanidin, Paeonidin (Peonidin), Delphinidin (Oenantidin), Petunidin, Malvidin. Anthocyanins must only be obtained from edible fruit or vegetables such as strawberries, mulberries, cherries, plums, raspberries, blackberries, black and red currants, red cabbage, red onions, cranberries, huckleberries, aubergines, grapes and elderberries. |
|
E 170 |
Calcium carbonate |
CaCO3 |
|
E 171 |
Titanium(IV)-oxide (Titanium dioxide) |
TiO2 |
|
E 172 |
Iron oxides and -hydroxides |
xFe2O3 · yFeO · nH2O |
|
E 173 |
Aluminum |
Al |
|
E 174 |
Silver |
Ag |
|
E 175 |
Gold |
Au |
For the substances with the EEC numbers E 102, E 104, E 110, E 122 to E 124, E 127, E 131, E 132, E 142 and E 151 the acid on which the compound is based and any sodium, calcium, potassium and aluminum salt of this substance islicensed in addition to the sodium or calcium salt of the substance which is given in the column “Chemical name orDescription”.
Synthetic colouring substances which are identical to the natural colouring substances mentioned are also authorized.
Preservatives
Preservatives by Frequency of Use in Cosmetic Formulations
The use of preservatives for cosmetic purposes is regulated in the EU by the Commission Directive 2007/17/EC of 22 March 2007, amending Council Directive 76/768/EEC, concerning cosmetic products, for the purposes of adapting Annexes III and VI.
eur-lex.europa.eu/Notice.do
The Annex VI of the Council Directive 76/768/EEC can be found under
borealischem.com/pdf/Annex6%20Parts1-2PreservativesList.pdf
In the US preservatives are registered by the FDA.
In Japan the use of preservatives in cosmetics is regulated by the “Standards for Cosmetics” (Ministry of Health and Welfare Notification No.331 of 2000), Appendix 3, see also
www.mhlw.go.jp/english/topics/cosmetics/index.html
The frequency of use of preservatives in the US in 2005 is given in the following table.
|
Preservative |
Frequency of use (2005) |
|---|---|
|
Methylparaben |
7866 |
|
Propylparaben |
6260 |
|
Butylparaben |
2784 |
|
Ethylparaben |
2310 |
|
Phenoxyethanol |
2227 |
|
Imidazolidinyl urea |
2036 |
|
Sodium sulfite |
1306 |
|
Resorcinol |
1176 |
|
DMDM hydantoin |
1062 |
|
Diazolidinyl urea |
737 |
|
Methylchloroisothiazolinone/methylisothiazolinone 818 |
699 |
|
Sorbic acid/potassium sorbate |
639 |
|
Benzoic acid/Sodium benzoate |
582 |
|
Dehydroacetic acid/Sodium dehydroacetate |
559 |
|
Quaternium-15 |
515 |
|
Isobutylparaben |
507 |
|
Benzyl alcohol |
502 |
|
Triclosan |
484 |
|
Boric acid/sodium borate |
353 |
|
Iodopropynyl butylcarbamate |
195 |
|
2-Bromo-2-nitropropane-1,3-diol |
187 |
|
Sodium methylparaben |
160 |
|
Salicylic acid |
140 |
|
Methyldibromo glutaronitrile |
115 |
|
Formaldehyde |
113 |
|
Benzalkonium chloride |
82 |
|
Chlorhexidine digluconate |
68 |
|
Sodium bisulfite |
62 |
|
Chlorphenesin |
58 |
|
Hexamidine isethionate |
47 |
|
Isopropylparaben |
47 |
|
Chloroxylenol |
44 |
|
Chloroacetamide |
36 |
|
Sodium hydroxymethylglycinate |
35 |
|
5-Bromo-5-nitro-1,3-dioxane |
34 |
|
Benzethonium chloride |
32 |
|
Sodium propylparaben |
30 |
|
Methenamine |
28 |
|
o-Phenylphenol/Sodium-o-phenylphenol |
28 |
|
Phenethyl alcohol |
28 |
|
Grapefruit Seed Extract |
23 |
|
Triclocarban |
22 |
|
Paraformaldehyde |
21 |
|
Glutaraldehyde (Glutaral) |
19 |
|
Polymethoxy bicyclic oxazolidine |
19 |
|
Chlorhexidine dihydrochloride |
18 |
|
o-Cymen-5-ol |
18 |
|
Thymol |
13 |
|
Piroctone olamine |
12 |
|
Dichlorobenzyl alcohol |
11 |
|
Chlorhexidine acetate |
9 |
|
Climbazole |
9 |
|
Formic acid and salts |
7 |
|
Hinokitiol |
7 |
|
Domiphen bromide |
6 |
|
Phenoxyisopropanol |
6 |
|
Dichlorophene |
5 |
|
p-Chloro-m-cresol |
5 |
|
Phenyl mercuric acetate |
5 |
|
Thiomersal |
5 |
|
Undecylenic acid and salts |
4 |
|
Dimethyloxazolidine |
2 |
|
Polyaminopropyl biguanide |
2 |
|
Benzylparaben |
1 |
|
Chlorhexidine |
1 |
|
7- Ethylbicyclooxazolidine |
1 |
|
Total formulations reported |
22228 |
Reference: D. C. Steinberg, 2005 Preservatives use: Frequency reports and registration, Cosmetic and Toiletries magazine 121(7), 65-69 (2006).
Emollients
Emollients by Frequency of Use in Cosmetics in USA
Cited from: D.C. Steinberg, Cosmet. Toiletries 112, No 6, 31 (1997)
|
Emollient |
Frequency of use (1996) |
|---|---|
|
Cetyl alcohol |
2860 |
|
Dimethicone |
1898 |
|
Mineral oil, heavy |
1847 |
|
Mineral oil, light |
1695 |
|
Carnauba wax |
1309 |
|
Beeswax, white |
1244 |
|
Isopropyl myristate |
1171 |
|
Stearyl alcohol |
1129 |
|
Cyclomethicone |
1056 |
|
Castor oil |
976 |
|
Octyldodecanol |
855 |
|
Cetearyl alcohol |
847 |
|
Lanolin |
838 |
|
Caprylic/Capric triglyceride |
774 |
|
Candelilla wax |
773 |
|
Petrolatum, yellow |
761 |
|
Ozokerite |
730 |
|
Polyethylene |
647 |
|
Lanolin oil |
646 |
|
Isopropyl palmitate |
616 |
|
Paraffin |
590 |
|
Squalane |
581 |
|
Petrolatum, white |
549 |
|
Octyl palmitate |
533 |
|
Japan wax |
513 |
|
Isopropyl lanolate |
494 |
|
Microcrystalline wax |
467 |
|
Oleyl alcohol |
438 |
|
Acetylated lanolin alcohol |
411 |
|
Hydrogenated vegetable oil |
406 |
|
Sweet almond oil |
399 |
|
Ceresin |
347 |
|
Wheat germ oil |
331 |
|
Jojoba oil |
330 |
|
C12-15 alcohols benzoate |
298 |
|
Sesame oil |
287 |
|
Cetearyl octanoate |
272 |
|
Hydrogenated cottonseed oil |
272 |
|
Coconut oil |
268 |
|
Cetyl palmitate |
263 |
|
Wheat germ glycerides |
259 |
|
Avocado oil |
249 |
|
Myristyl myristate |
249 |
|
Polybutene |
241 |
|
Corn oil |
231 |
|
Myristyl lactate |
224 |
|
Cetyl acetate |
218 |
|
Cetyl esters wax |
217 |
|
Trilaurin |
216 |
|
Hydrogenated castor oil |
211 |
|
Shea butter |
208 |
|
Propylene glycol dicaprylate/caprate |
202 |
|
Synthetic jojoba oil |
199 |
|
Beeswax, yellow |
196 |
|
Decyl oleate |
187 |
|
Acetylated lanolin |
183 |
|
Hydrogenated polyisobutene |
164 |
|
Synthetic beeswax |
156 |
|
Cocoa butter |
151 |
|
Hydroxylated lanolin |
144 |
|
Soybean oil |
141 |
|
Hydrogenated lanolin |
133 |
|
Mink oil |
131 |
|
Sunflower oil |
131 |
|
Safflower oil |
128 |
|
Stearyl heptanoate |
128 |
|
Macadamia nut oil |
127 |
|
Olive oil |
124 |
|
Diisostearyl malate |
121 |
|
Hydrogenated tallow |
120 |
|
Apricot kernel oil |
119 |
|
Lanolin wax |
118 |
|
Hazelnut oil |
105 |
|
Octyl hydroxystearate |
104 |
|
Isopropyl isostearate |
103 |
|
Borage oil |
101 |
Surfactants
Table of Contents
1 Anionic Surfactants
- 1.1 Salts and Esters of Carboxylic Acids
- 1.2 Sulfuric Acid Derivatives
- 1.3 Sulfonic Acids and Salts
- 1.4 Phosphoric Acid Esters and Salts
- 1.5 Acylamino Acids and Salts
2 Cationic Surfactants
2.1 Alkyl Amines
- 2.2 Alkylimidazolines
- 2.3 Quaternary Ammonium Compounds
- 2.4 Ethoxylated Alkyl Amines
- 2.5 Esterified Quaternaries
3 Amphoteric Surfactants
- 3.1 Acyl Ethylenediamines and Derivatives
- 3.2 N-Alkyl Amino Acids or Imino Diacids
- 3.3 Alkyl Betaines
4 Nonionic Surfactants
- 4.1 Fatty Alcohols
- 4.2 Ethers
- 4.3 Alkanolamides
- 4.4 Esters
- 4.5 Ester/Ether Surfactants
- 4.6 Amine Oxides
5 Alkoxylated Polysiloxanes
6 Fluorosurfactants
Comparison of the Properties of Various Surfactant Types
Surfactants, Preferably Used in the Manufacture of Pharmaceuticals and/or Cosmetics
The classification given below is a modified version of a review presented by L. O. de Guertechin (in G. Broze, Handbook of Detergents (Surfactant Series Vol. 82), Part A: Properties, pp. 7–46, Marcel Dekker Inc., New York and Basel 1999).
1 Anionic Surfactants
1.1 Salts and Esters of Carboxylic Acids
1.1.1 Carboxylic Acid Salts (Soaps)
Free fatty acids are not used as surfactants, due to their low water solubility. However water-soluble fatty acid salts like alkali and short-chain amine salts (ethanol amine, diethanol amine, triethanol amine) show good water affinity and are widely used. Saturated sodium soaps are extremely soluble in water up to C8 (these are not yet true surfactants); they become less soluble up to C18 (i.e., the domain of effective surfactants) and insoluble above C20. The fatty acids can be either saturated or unsaturated, starting from C16chain lengths. Unsaturated fatty acids are prone to undergo oxidation and form oxides and peroxides which cause rancidity and yellowing. Potassium soaps and salts of alkanolamines are more fluid and also more soluble than sodium salts.
|
|
Fatty acid alkali salts (R = C7H15 −C17H35)
|
|
Fatty acid earth alkali salts (R = C7H15 −C17H35)
|
|
Fatty acid ethanol amine salts (R = C7H15 −C17H35)
|
|
Fatty acid isopropanol amine salts (R = C7H15 −C17H35)
Applications.
The main application of fatty acid salts is found in the soap bars used worldwide for hand-washing fabrics (generally based on tallow/coconut oil mixtures). Water-soluble soaps are mainly used in skin cleansers (soap bars or liquids), shaving products (sticks, foams, or creams), and deodorant sticks. Water-insoluble soaps form gels in nonaqueous systems and, due to their hydrophobicity, they can be appropriate surfactants or thickeners for w/o emulsions. Some of them are used as lubricants (e.g. magnesium stearate and calcium arachinate).
1.1.2 Ester Carboxylic Acids
They are monoesters of di- and tricarboxylic acids. These esters are produced by condensation reactions involving different types of molecules: either an alcohol with a polycarboxylic acid (e.g., tartaric or citric acid), or an hydroxyacid (e.g., lactic acid or citric acid) with a carboxylic acid. The alcohol may have been previously ethoxylated to enhance water solubility and surface activity.
|
|
Sodium dilaureth-7-citrate
|
|
Stearoyl disodium tartrate
Applications.
Due to their good foaming properties and substantivity on the hair, ester carboxylates are especially suitable in shampoos; in combination with alcohol ethoxy sulfates, they reduce skin irritation.
1.1.3 Ether Carboxylic Acids
These surfactants are formed by the reaction of sodium chloracetate with ethoxylated alcohols. Due to the addition of ethoxylated groups, ether carboxylates are more soluble in water and less sensitive to water hardness compared to conventional soaps. Also, keeping the best properties of nonionic surfactants, they do not exhibit any cloud point and show good wetting and foam stability. Ether carboxylates do not undergo hydrolysis in the presence of alkalis or acids.
|
|
Alkyl polyglycol ether carboxylate, sodium salt (R = C8H17 −C18H37)
Applications.
Emulsifiers and emulsion stabilizers, in hair conditioners and in shampoos in combination with alcohol ether sulfates and possibly with cationics.
1.2 Sulfuric Acid Derivatives
1.2.1 Alkyl Sulfates
Alkyl sulfates are organic esters of sulfuric acid; the sulfur atom is bridged to the carbon atom of the hydrocarbon chain via an oxygen atom. Sodium lauryl sulfate (SLS), one of the most common surfactants, belongs to this class.
|
|
Sodium alkylsulfate (Ropt. = C12H25 −C14H29)
|
|
Ammonium alkylsulfate (Ropt. = C12H25 −C14H29)
|
|
Monoethanol amine alkylsulfate (Ropt. = C12H25 −C14H29)
Applications.
Alkyl sulfates have been, for about 25 years, the most important synthetic surfactant. They are foamers, emulsifiers and are still used in cosmetics and personal care areas. They are also used in combination with other surfactants to improve the foaming characteristics of detergent systems. Pure SLS is used in oral care and incorporated in dental creams.
1.2.2 Alkyl Ether Sulfates
Alkyl ether sulfates (AES), which are also called alcohol ethoxy sulfates (AEOS), result from the sulfation of an ethoxylated alcohol.
|
|
Sodium alkyl ether sulfate
Applications.
Alkyl ether sulfates are used as household cleaners (e.g., carpet cleaners), dishwashing liquids, and fabric care (powders and liquids), in personal care products such as liquid soaps, shower gels, foam baths, and, especially, shampoos. Increasing ethoxylation degree reduces skin and eye irritation. They are generally combined with other nonionic or anionic surfactants.
1.3 Sulfonic Acids and Salts
In contrast to alkyl sulfates in alkyl sulfonates the sulfur atom is directly linked to the carbon atom making the substances stable against hydrolysis.
1.3.1 Alkyl Sulfonates
Three major types of alkyl sulfonates must be considered: the primary and secondary paraffin sulfonates (PS and SAS) and the α-olefin sulfonates (AOS).
|
|
Primary sodium alkyl sulfonate
|
|
Secondary sodium alkyl sulfonate
|
|
Sodium alkene sulfonate
|
|
Sodium hydroxy alkane sulfonate
Applications.
Alkane sulfonates (PS and SAS) are very water soluble showing good foaming, wetting and emulsifying properties. They are mainly used in Europe in heavy- and light-duty powder detergents as well as in all-purpose hard-surface liquid cleaners. Due to their excellent resistance to high electrolyte contents, alkane sulfonates have also found interesting prospects in concentrated industrial or domestic cleaners containing mineral chemical additives.
α-Olefin sulfonates have been mainly used in Asia as surfactants for heavy- and light-duty laundry detergents, synthetic soap bars, and household products; they have also been used in the United States in several personal care products (liquid soaps, bubble baths, and shampoos) as alternatives to alcohol ether sulfates. They are also marginally used in oral care formulations.
1.3.2 Alkyl Aryl Sulfonates
Linear alkylbenzene sulfonates are the most important surfactants used. They are biodegradable. Linear alkylbenzene sulfonates exhibit good chemical and thermal stabilities and can be incorporated in spray-dried slurries.
|
|
Sodium linear alkylbenzene sulfonate (LAS) (R = C9H19 −C15H31)
Applications.
Linear alkylbenzene sulfonates are very cost-effective surfactants which are used in a broad variety of detergents for household, fabric care, institutional, and industrial products. In laundry products (powders and liquids), LAS is the surfactant of choice, usually used in combination with other anionic or non-ionic surfactants. LAS is also an appropriate anionic surfactant for light-duty and delicate powder laundry detergents. Linear alkylbenzene sulfonates are wellknown in hand dishwashing formulations, often in combination with AEOS (i.e., alcohol ethoxy sulfate), providing better foam resistance. Due to its very high detersive action, LAS has a low compatibility with skin and is scarcely used in cosmetics, except in antiseborrheic preparations.
1.3.3 Sulfosuccinates
Sulfosuccinates are the sodium salts of alkyl esters of sulfosuccinic acid; monoesters of sulfosuccinic acid based on linear fatty alcohols are only partially water-soluble and hardly dispersible. Those based on fatty alcohol ethoxylates exhibit much better solubility. Dialkyl esters based on alcohols with less than nine carbons, preferably five to eight carbon atoms, as well as those based on fatty acid ethanol amides are water soluble and, therefore, are generally preferred. Disodium salts of monoesters deliver good detergency and foam properties. Due to the ester linkage, all sulfosuccinates are sensitive to hydrolysis, especially under acidic conditions.
|
|
Sodium dialkyl sulfosuccinate
|
|
Sodium alkyl sulfosuccinamate
|
|
Sodium ethoxylated alkylamido sulfosuccinate
Applications.
The monoesters of alkanolamides and their derivatives are extensively used in personal care products and especially in shampoos often in combination with other anionic surfactants. The diesters are used as dispersing and wetting agents in industrial or institutional applications such as emulsion polymerization, the textile industry, ink manufacture, dry cleaning, and agriculture.
1.3.4 Sulfo Fatty Acid Esters
Among this group of surfactants the α-sulfo fatty acid esters are commonly used on an industrial scale. The α-sulfo fatty acid esters contain the sulfonate group statistically distributed along the carboxylate chain.
|
|
Alkyl ester of α-sulfo fatty acid sodium salt
Applications.
α-sulfo methyl ester surfactants deriving from C16-C18 fatty acid are used in phosphate-free laundry detergents.
1.3.5 Fatty Acid Isethionates and Taurides
Taurides (or taurates) are acylamino alkane sulfonates which have chemical structures close to isethionates.
|
|
Fatty acid isethionate
|
|
Tauride
Applications.
These surfactants are insensitive to water hardness and show good wetting, foaming, and emulsifying properties. In addition, they have excellent compatibility with the skin. Acyl isethionates have been used in shampoos and personal cleaners. They are also incorporated in syndet bars, together with various soaps.
1.4 Phosphoric Acid Esters and Salts
This class of surfactants includes alkyl phosphates and alkyl ether phosphates.
|
|
Disodium alkyl phosphoric ester
|
|
Sodium dialkyl phosphoric ester
|
|
Sodium ethoxylated alkyl phosphate
|
|
Sodium di-ethoxylated alkyl phosphate
Applications.
Phosphate esters are used in formulations where a particular tolerance to pH, heat, or electrolytes is required. They are also used in acidic cleaning products for household as well as industrial applications. They act as metal stripping or dipping agents and, thereby, increase paint adhesion. The phosphate esters also show antistatic properties to the treated substrates. Incorporated in dry cleaning compositions, phosphate ester provide, in addition to an exceptional detergency. The less water-soluble phosphate esters are also used as antifoaming agents and are applied as emulsifiers in agrochemical applications (e.g., concentrate fertilizer solutions).
1.5 Acylamino Acids and Salts
1.5.1 Acyl Glutamtes
Acyl glutamtes are based on α-aminoglutaric acid.
HOOC−CH2−CH2−CH(NH2)−COOH
α-aminoglutaric acid
|
|
Sodium acylglutamate
Applications.
Personal care products such as shampoos, mild to the skin and delivering improved skin feel.
1.5.2 Acyl Peptides
Acyl peptides are formed from hydrolyzed proteins e.g., animal collagen. The average polypeptide molecular weight can vary from about 350 to 2000.
|
|
Sodium acyl polypeptide(X = amino acids side groups)
Applications.
Acyl peptides are used in shampoos, they are prone to microbial degradation and are rather tolerant to water hardness.
1.5.3 Acyl Sarcosides
Sarcosinates (or salts of acyl amino acids) are the condensation products of fatty acids with N-methylglycine (CH3-NH-CH2-COOH) (or sarcosine).
|
|
Acylamino acid sodium salt
Applications.
Sarcosinates are mild to skin. They are also used as corrosion inhibitors.
2 Cationic Surfactants
2.1 Alkyl Amines
Primary, secondary, and tertiary alkyl amines and, especially, their salts are uncharged in neutral solution and therefore are not strictly cationic. They can be considered as cationics in a pH low enough to provide the ionic form; otherwise, they must be considered as nonionics. Salts of fatty amines can deliver a germicidal activity; their fungicidal efficacy is enhanced when the amine is neutralized with salicylic or o-chlorobenzoic acid.
|
|
Alkyl amine salt
|
|
Dimethyl alkyl amine salt
|
|
Alkylamido dimethyl propylamnine salt
Applications.
Amines are used in textile treatment (e.g., antistatic treatment) and occasionally in rinse fabric softeners. Amido-amines are also used in cosmetic products. Salts of stearyl and tallow fatty amines are used in some mining applications (e.g., flotation process). Fatty amines, diamines and polyamines find other prospects as adhesive agents in the coating of damp surfaces with paint or bitumen and as corrosion inhibitors.
2.2 Alkylimidazolines
|
|
Alkyl aminoethyl imidazoline
|
|
Alkyl hydroxyethyl imidazoline
Imidazolines are cationic O/W emulsifiers. They adsorb at metal surfaces and improve the adhesion of the applied layer to substrates.
2.3 Quaternary Ammonium Compounds
2.3.1 Tetraalkyl(-aryl) Ammonium Salts
|
|
The water solubility of quaternaries primarily depends on the nature of R substituents (hydrophobic chain lengths, polarity, etc.). Quaternaries carrying two or more long hydrophobic chains have very poor water solubility. Low-solubility quaternaries can adsorb on various substrates and impart various useful conditioning effects (softening, antistatic, corrosion inhibition, etc.). Quaternaries are generally not compatible with anionics because of the formation of a water-insoluble complex.
Applications.
The major use of quarternaries is related to their ability to adsorb on natural or synthetic substrates and fibers. Less-soluble long hydrophobic chain containing compounds (e.g., C16-C18 dialkyldimethyl ammonium chlorides) deposit on fibers. Their softening and antistatic properties are similarly exploited in hair conditioning shampoos or after-shampooing rinses. In cosmetic applications, quaternaries could cause ocular and local irritation; nevertheless, their potential for skin penetration is very low. Among the quaternaries, some are used as germicides, disinfectants, or sanitizers, they are especially effective against gram-positive but less effective against gram-negative bacteria. Quaternaries are also used as emulsifiers in acidic creams and lotions. N-Alkyltrimethyl ammonium salts are used as emulsifiers in applications requiring a selective adsorption of the emulsifier on the treated substrate.
2.3.2 Heterocyclic Ammonium Salts
Heterocyclic quaternaries are derived from heterocyclic aliphatic or aromatic compounds. They are often based on morpholine, imidazoline, pyridine and isoquinoline.
|
|
Alkylethyl morpholinium ethosulfate
|
|
Alkylpyridinium chloride
|
|
Dialkylmethyl imidazolinium methosulfate
Applications.
The quaternaries derived from imidazoline and morpholine are used as hair conditioners and antistatic agents. Those derived from aromatic heterocycles are used as germicides. N-Alkyl imidazoline chlorides are also used as emulsifiers in applications where the adsorption of the emulsifying agent on the substrate is desired.
2.4 Ethoxylated Alkyl Amines
These surfactants can be considered as cationic or nonionic, depending on the degree of ethoxylation and on the pH at which they are used. Polyethoxylated amines are formed by ethoxylation of primary or secondary fatty amines. The poloxamines are formed by the reaction of ethylene diamine with propylene oxide. Other tetrafunctional products are obtained by successive reactions of ethylene diamine with ethylene oxide and propylene oxide. lt must be noted that the above surfactants based on ethylene diamine, although intrinsically cationic, essentially behave as nonionic surfactants.
|
|
Laurylamine-POE-6
|
|
Ethoxylated diamidoamine chloride
|
|
Alkyl propanediamine ethoxylate
|
|
Ethylene diamine based POE/POP product
Applications.
The ethoxylated alkyl amines are emulsifying agents in agrochemical emulsions, wax emulsions, and two-phase emulsion cleaners. They are used as corrosion inhibitors in oil refineries. In personal care, ethoxylated alkyl amines act as emulsifiers and hair conditioning agents.
2.5 Esterified Quaternaries
Esterified quaternaries (or esterquats) show fabric softening properties. They are biodegradable and nonsensitizing agents which could be used in dermatology.
|
|
N-Methyl-N,N-bis-[(cetostearoyl)ethyl]-N-(2-hydroxyethyl)ammonium-methosulfate
Applications.
The esterquats are suitable substitutes for straight quaternaries with comparable softening properties.
3 Amphoteric Surfactants
Amphoteric surfactants show depending on the pH of the solution a positive or a negative charge. They exhibit to have a ”zwitterionic” character showing an isoelectric point.
3.1 Acyl Ethylenediamines and Derivatives
These surfactants show amphoteric properties and the zwitterionic form appears around neutral pH; the water solubility is minimal at the isoelectric point.
|
|
N-Hydroxyethyl-N-carboxymethyl-N’-acyl-ethylenediamne sodium salt
|
|
N-Carboxymethyl-N-carboxymethyl-oxyethyl-N’-acyl-ethylenediamine disodium salt
|
|
N-Carboxyethyl-N-carboxyethyloxyethyl-N’-acyl-ethylenediamine disodium salt
Applications.
Amphoterics of this class are similar to those of betaines. They are used in personal care products, baby shampoos, fabric softeners, industrial and car cleaners. They are compatible with other surfactants and tolerate hard water and electrolytes.
3.2 N-Alkyl Amino Acids or Imino Diacids
These molecules are chemical derivatives of amino acids.
|
|
Alkyl aminopropionic acid sodium salt
|
|
Sodium coco glycinate
|
|
Di-carboxyethyl-alkylamin disodium salt
Applications.
N-Alkyl amino acids or diacid amphoterics are used in personal care household products. They are compatible with other surfactants, electrolytes and hard water. They show good emulsifying, foaming and wetting properties.
3.3 Alkyl Betaines
The positive charge is always carried by a quaterized nitrogen, whereas the anionic site can be a carboxylate (betaine), a sulfate or a phosphate. Betaines are good foaming, wetting, and emulsifying surfactants, especially in the presence of anionics. Detergency is best in alkaline conditions. Betaines are compatible with other surfactants and they frequently form mixed micelles; these mixtures often deliver unique properties which are not found in the individual constitutive surfactants. In their straight cationic form (i.e., in neutral and acidic conditions), betaines are not affected by water hardness ions and other metallic ions. They have hydrotropic properties, helping to solubilize ethoxylated nonionic surfactants in the presence of salting-out ions. Betaines are especially mild to skin and have the ability to improve the skin tolerance against irritating anionic surfactants.
|
|
Alkylbetaine
|
|
Alkylamidopropyl betaine
|
|
Imidazolinium betaine
|
|
Alkylamidopropyl hydroxysultaine
|
|
Alkylamidopropyl hydroxyphostaine
Applications.
Betaines have low eye and skin irritation; moreover, the presence of betaines is known to decrease the irritation effect of anionics. For the above reasons and also due to their high price, they are usually used in association with other surfactants. Betaines are thus especially suitable in personal care applications (shampoos, foam baths, liquid soaps, shower gels, etc.), fabric hand-wash products, and dish-washing products.
4 Nonionic Surfactants
4.1 Fatty Alcohols
|
|
Applications. Fatty alcohols are primarily used as co-emulsifiers. They are starting materials in the production of ethoxylated fatty alcohols.
4.2 Ethers
4.2.1 Ethoxylated Fatty Alcohols
There is a wide range of emulsifiers, wetting agents and solubilizers based on fatty alcohols available. They vary in both: the chain length of the fatty alcohol and the ethoxyl content. The influence of the fatty alcohol chain length on the properties of the compound is small compared to that of the polyoxyethylene chain.
|
|
Fatty alcohol polyglycol etherwith x = C8 −C18 and y =∼ 2 to 300
Applications.
Detergents in industrial and household products often in combination with nonionics. Ethoxylated fatty alcohols are also used in personal care products as emulsifiers and solubilizers.
4.2.2 Ethylene oxide/Propylene oxide-Block Polymers
These surfactants consist of a central polypropylene glycol part (PPG) representing the hydrophobic portion of the molecule and two hydrophilic polyethylene glycol chains (PEG). They are also called EO/PO block polymers. Depending on the mol weight and the proportion between PPG and PEG a wide variety of surface active agents can be produced, showing different properties.
|
|
Applications.
EO/PO block polymers are used in dishwashing and laundry detergents. They have thickening and gelling properties which makes them interesting for cosmetics. In the pharmaceutical field the more hydrophilic types are used as solubilizers under the name poloxamer.
4.2.3 Alkylphenol Ethoxylates
They are also called alkylphenol polyglycol ethers and belong to the most important types of washing active substances with excellent wetting properties. Due to ecotoxicological reasons their use however diminishs. The octylphenol and nonylphenol ethers are of special importance.
|
|
Octylphenol polyglycol ether
|
|
Nonylphenol polyglycol ether
Applications.
Depending on the degree of ethoxylation they may be used as wetting and washing agents as well as emulsifiers and solubilizers.
4.2.4 Alkylpolyglucosides
The numerous hydroxyls of glucoside groups ensure the solubility of the whole molecule in water. Alkylpolyglucosides (APGs) show good water solubility and have their cloud points at rather high temperatures (generally above 100℃); they are only slightly sensitive to the presence of electrolytes and are only very rarely influenced by water hardness. The optimal detergency of APGs is found for an average alkyl chain length around 13 and a glucosidic content of about 65 %. Alkylpolyglucosides show good chemical stability at neutral and alkaline pH. Their biodegradability is excellent.
|
|
AlkylpolyglucosideR = fatty acid
Applications.
Alkylpolyglucosides are used in detergents and personal care cleansers (e.g., shampoos); they are known to be mild for skin.
4.2.5 Ethoxylated Oils and Fats
This class of surfactants covers ethoxylated derivatives of lanolin (wool fat) and castor oil. Lanolin is the generic name of a wax containing a complex mixture of esters and polyesters of high-molecular-weight alcohols (aliphatic, steroid, and triterpenoid) and fatty acids (saturated, unsaturated, hydroxylated, and nonhydroxylated). Castor oil is the natural extract resulting from cold pressing of ricinus seeds, a mixture of a triglyceride of fatty acids (ricinoleic 87.5 %, oleic 5 %, linoleic 4 %, palmitic 1.5 %, linolenic 0.5 %, stearic 0.5 %, dihydroxystearic 0.5 % and arachidic 0.5 %).
Applications.
Ethoxylated products of lanolin and castor oil are good emulsifiers and solubilizers respectively. They are mainly used in the cosmetics industry; the ricinus based products are solubilizers for pharmaceutical purposes.
4.3 Alkanolamides
4.3.1 Alkanolamides
Alkanolamides are N-acyl derivatives of monoethanolamine and diethanolamine.
|
|
Monoalkanolamide
|
|
Dialkanolamide
|
|
Esteramide
Applications.
Alkanolamides are foamers, foam boosters and foam stabilizers. They are used in household detergent products, shampoos and cleaners.
4.3.2 Ethoxylated Alkanolamides
The reaction of an alkanolamide with ethylene oxide leads to an ethoxylated amide. Their properties are comparable to those of ethoxylated alcohols.
|
|
Polyethoxylated monoalkanolamide
|
|
Polyethoxylated dialkanolamide
Applications.
As thickening, foam stabilizing and dispersing substances. Their application in car wash is also mentioned.
4.4 Esters
4.4.1 Ethoxylated fatty acids
Theoretically POE could react at both ends with one fatty acid. Nevertheless the reaction of a fatty acid with ethylene oxide leads mainly to the monoester with a broad distribution in the molar EO number (n); the reaction of polyethylene glycol with fatty acids leads to a mixture of mono- and diesters besides free fatty acid.
|
|
Fatty acid monoester
|
|
Fatty acid diester
The monoesters are much more soluble in water than the diesters. These surfactants are readily hydrolyzed under acidic or alkaline conditions.
Applications.
Fatty acid POE esters are excellent emulsifiers for cosmetic, household and indurial use.
If properly balanced, combinations of esters with low and high degree of ethoxylation provide excellent emulsifier properties for creams and lotions. Therefore they have special interest in combined emulsifiers in pharmacy.
4.4.2 Glycol and Glycerol Esters
The esters of fatty acids and glycol or glycerol are lipopholic surfactants showing a HLB value below 10.
|
|
Ethylene glycol ester
|
|
Propylene glycol ester
|
|
Glycerol monoester(Monoglyceride)
|
|
Glycerol-1,3-diester(Diglyceride)
Applications.
Due to their very low toxicity, these surfactants are edible and, therefore, they are widely used in the food industry, especially in applications involving W/O emulsions or dispersions (e.g., butter, diet butter, margarine, etc.). Glycol and glycerol esters are also used in pharmaceutical and cosmetic industry either as emulsifying agents or as oily compounds in creams, lotions, ointments, gels.
4.4.3 Sorbitan Esters
Sorbitol can form two different sorbitans by internal dehydration: the 1,4- and the 1,5-sorbitan. Further dehydration of the 1,4-sorbitan leads to iso-sorbide. In common sorbitan based surfactants the 1,4-sorbitan is dominating. Fatty acid esters of sorbitan are insoluble in water, their HLB value is below 10.
Chemical structure of the basic compounds
|
|
Sorbitol
|
|
1,4-sorbitan
|
|
1,5-sorbitan
|
|
iso-sorbide
Chemical structure of sorbitan based emulsifiers
|
|
1,4-Sorbitan monoester
|
|
1,4-Sorbitan triester
Applications.
Emulsifiers often in combination with ethoxylated sorbitanesters of fatty acids.
4.4.4 Alkyl Carbohydrate Esters
Alkyl carbohydrate esters are also known under the names ”sugar esters” and”sucrose esters”. Normally they ar based on saccharose. Mono- and diesters are produced. The mono esters are water soluble while the dieseters are not. Due to the steric effects, primary hydroxyl groups are almost exclusively subject to esterification. They are of great interest due to their natural origin of their components and good biodegradability.
|
|
Saccharose fatty acid monoester
|
|
Saccharose fatty acid diester
Applications.
Sucrose esters are food-grade ingredients and have similar food additive usages as the previously described glycol, glycerol, and sorbitan esters.
4.5 Ester/Ether Surfactants
These surfactants contain besides the ester group, which is formed by a fatty acid and a polyol as described in the previous section, ether linkages normally originating from polyoxyethylene. In general they are more hydrophilic compared to the ester surfactants and exhibit HLB values above 10.
4.5.1 Ethoxylated Glycol and Glyerol Esters
|
|
Ethoxylated glycol monoester
|
|
Ethoxylated glycerol monoester
Besides the monoesters diesters are possible mainly in the case of glycerol.
Applications.
Emulsifiers for O/W emulsions in cosmetic and pharmacy.
4.5.2 Ethoxylated Sorbitan Esters
Ethoxylation of sorbitan fatty acid esters leads to an important surfactant class for pharmacy and cosmetics. The fatty acids used are: lauric, myristic, palmitic, stearic and oleic acid.
|
|
Ethoxylated 1,4-sorbitan monooleate (as an example) w + x + y + z≅20
Applications.
Emulsifiers for cosmetic and pharmaceutical emulsions, solubilizers for oily liquids like vitamins and hormones. Wetting agents in suspensions.
4.5.3 Ethoxylated Pentaerythritol Esters
|
|
Ethoxylated pentaerythritol monoleate
Applications.
Emulsifier for cosmetic preparations.
4.5.4 Polyglycerol monoester
This is the only class of ether bounds containing emulsifiers which do not contain polyoxyethylene groups. The ether linkage is formed from glycerol units.
|
|
Polyglycerol monoleate
4.6 Amine Oxides
Amine oxides are produced from alkyl dimethyl amine, where the alkyl chain is a C12-C18 unit by oxidation. The methyl groups could be partially replaced by other functional groups like amidopropyl, hydroxyethyl or hydroxypropyl.
|
|
Alkyl dimethylamine oxide
Applications.
Incorporated in shampoos, amine oxides contribute to impart viscosity, reduce eye irritancy, and enhance foam properties. They are especially suitable in slightly acidic or neutral formulas. In domestic cleaners the amine oxides are used in association with anionics. Industrial applications are: liquid bleach products, textile industry, foam stabilizers and anti corrosion formulations.
5 Alkoxylated Polysiloxanes
Alkoxylated polysiloxanes are derived from polydimethyl siloxane, where methyl groups are substituted by hydrophilic groups which can be anionic, cationic or nonionic.
|
|
A denotes a hydrophilic group like polyoxyethylene, amines etc.
Silicone surfactants show excellent wetting capacity on low-energy surfaces. Also, their diluted solutions can considerably decrease the surface tension to below 20 mN/m. Foam is easily controlled; foaming efficacy is depressed along with decreasing ethylene oxide/propylene oxide ratio. Ethoxylated polydimethylsiloxanes are rather inert and exhibit excellent chemical stability.
Applications.
These surfactants are used as additives in paints, foam controlling agents, textile auxiliaries, wetting agents and in cosmetic preparations like protectic creams, body milks, shampoos and conditioners.
The CFTA adopted name for these surfactants is Dimethicone copolyol.
6 Fluorosurfactants
Fluorosurfactants contain perfluoroalkyls chains F-(CF2-CF2)-, in which n ranges from about 3 to about 8. They are excellent wetting agents showing a critical surface tension of about 25mN/m. Similar to conventional surfactants, a rather broad variety of hydrophilic functions (ethoxylated chains, sulfonates, quaternaries, betaines, etc.) can be born by fluorosurfactants. These hydrophilic groups are generally not directly grafted on the fluorocarbon chain and are linked through a short intermediate hydrocarbon chain. Depending on their nature, these surfactants show variable emulsifying and foaming characteristics. They have excellent thermal and chemical stability; therefore, they find prospects in those extreme conditions in which the hydrocarbon surfactants would decompose. The major drawback of fluorosurfactants is that they are not environment friendly because they are resistant to biodegradation.
|
|
|
|
|
|
|
|
|
|
|
|
Applications.
Although they have some potential prospects in personal care (improved hair conditioning) and household, fluorosurfactants find their major applications in industrial areas like water based adhesives, wetting agents, flotation processes and battery technology.
Comparison of the Properties of Various Surfactant Types[1]
|
Properties |
Amphoteric |
Anionic |
Nonionic |
Cationic |
|---|---|---|---|---|
|
Foaming |
good |
good |
moderate to weak |
moderate to good |
|
Wetting |
weak |
good to very good |
good |
weak |
|
Emulsification |
good |
good |
good to very good |
weak |
|
Compatibility with |
||||
|
Electrolytes |
very good |
weak to good |
weak |
weak |
|
Acids |
very good |
weak |
weak |
good |
|
Bases |
very good |
weak |
weak |
weak |
|
Hardness Constituents of Water |
very good |
good |
moderate to weak |
moderate to weak |
|
Other Surfactant Types |
compatible with cationics |
incompatible with cationics |
compatible with all types |
incompatible with anionics |
|
Detergency |
good |
good |
good |
weak |
|
Irritation of Skin and/or Mucosa |
weak |
weak to strong |
weak to strong |
weak to strong |
|
Hydrotropic Effect |
very good |
weak to good |
not present |
not present |
|
Biodegradable |
yes |
not all types |
not all types |
not all types |
|
Lime soap dispersing |
very good |
good |
very good |
not compatible |
|
Synergistic Effect[a] |
very good with nonionics, anionics and cationics |
not generally |
good to very good with anionics and amphoterics |
not present |
|
Compatibility with soap foam |
very good |
defoaming action |
defoaming action |
incompatible |
|
|
|
|
|
|
|
[a] Refers to the ability to show enhanced properties in the presence of other surfactants. |
||||
[1] Individual representatives of the surfactant groups show deviating properties.
Available languages
Last update
Do you have any questions?
If you have any questions, please do not hesitate to contact us. The DrugBasePlus team will be happy to help you.
+49 711 2582-347
from 9.00 a.m. to 4.30 p.m., Fridays from 9.00 a.m. to 3.30 p.m.
































































































